

How can we say with resolve that AVINENT is synonymous with 100% quality? How do we know that our products are so totally safe and comply with all the established standards and requirements? Basically because, for AVINENT, quality is not only an aspect that needs to be controlled during the final phase in the manufacture of the product, but rather, quality is manufactured directly.
And it is this assurance of quality and regulatory matters that form the basis of AVINENT’s existence. The company tirelessly cares for all the aspects associated with quality, starting from the manufacturing processes, continuing with the safety and functionality of the product and to the facilities or the specific regulations of each country. Therefore, when an AVINENT product reaches a clinic, laboratory or hospital anywhere in the world, its guarantee of quality remains intact.
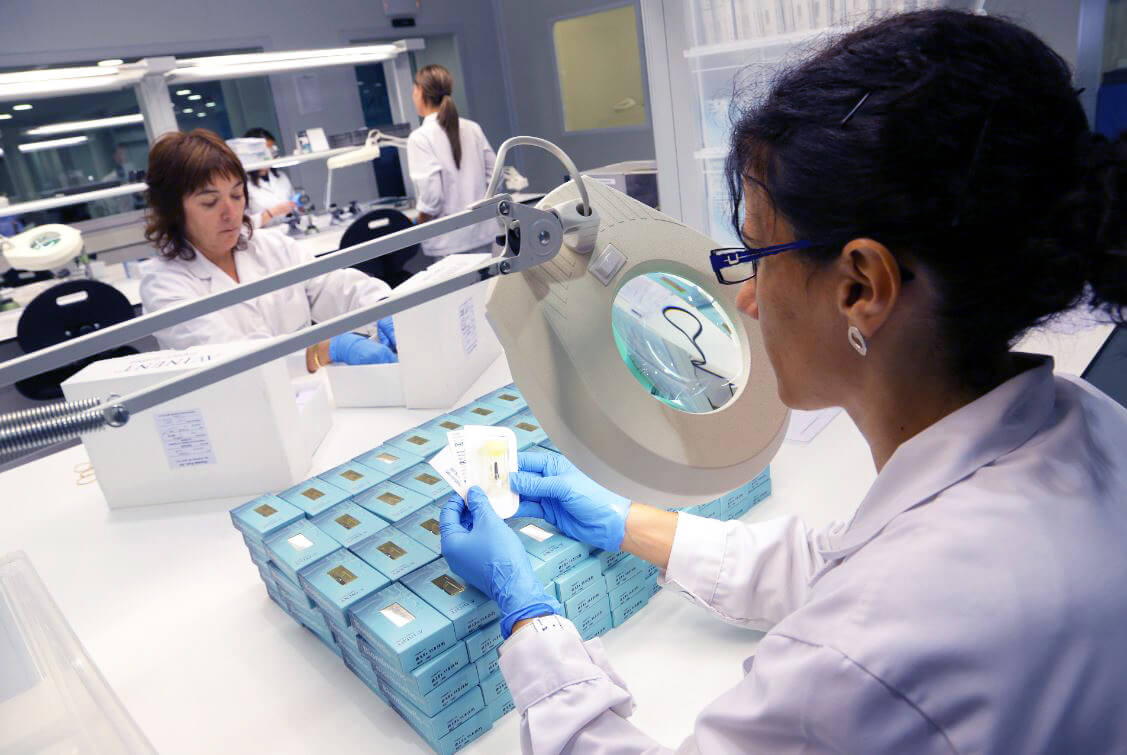
When we speak of quality in manufacturing processes, we are talking about ISO 13485:2016, the standard for companies manufacturing healthcare products. This quality certificate is renewed every three years, with the performance of annual follow-up audits, which in our case are carried out by notified body TÜV Rheinland. ISO 13485:2016 and the audits are essential elements in order to be able to manufacture. ISO covers the whole manufacturing process of the product, which includes both the assessment the company carries out of its suppliers and managing client traceability, including quality control during the production phase.
A second section on quality looks at the safety and functionality of the product, which is guaranteed in this case by the CE marking. This is a seal that gives a sense of security to a specific product, whether medical or not. Presently, and up until 2020 this standard is being reviewed and the current Directive 93/42 EEC will be replaced by the MDR 2017/745 (Medical Device Regulation), towards which AVINENT is already adapting. The CE marking is supported by a series of documentation and technical files which, in the case of AVINENT, cover the different product lines of the AVINENT Implant System, AVINENT CAD CAM and AVINENT Digital Health.
The task of the auditor is to carry out an annual review of one of the technical files of the company. This technical documentation is prepared by the AVINENT Regulatory Affairs team. The technical file contains the information on the manufacturer, product, families, packaging, etc. This file also includes the clinical assessment part, in which (increasingly more often) the company is required to have scientific proof, clinical follow-up and post marketing. It is this point where the role of universities and research centres play a vital role and it is here that AVINENT finds all the necessary support to carry out the scientific studies. AVINENT is currently actively promoting the creation of new scientific publications.
The third section referring to quality discusses the facilities. The company has a license for each product line, therefore, it holds a licence for the serial product AVINENT Implant System (granted by AEMPS) and for the personalised product AVINENT CAD CAM and AVINENT Digital Health, both granted by the Health Authority of the Autonomous Community of Catalonia.
Lastly, the fourth point covers the requirements that AVINENT must exceed at an international level, given that each country usually has specific requirements. In the United States for example, it is necessary to submit technical documentation to market a product. In this case it is the Food and Drug Administration, more commonly known as the FDA, which reviews the documentation and approves whether the company can commence commercial activities. AVINENT also has the necessary certificates to operate in other countries, such as, Canada, Australia, Japan, Taiwan, Mexico and Colombia.